
 Top of Part 1
Top of Part 1

 Last page
Last page

 Next page
Next page
  Top of Part 1
Top of Part 1
  Last page
Last page
  Next page
Next page
|
1-7: The Discovery of the Electron |
 [Vacuum Discharge, [Vacuum Discharge,
 the Cathode Rays] the Cathode Rays]

 Put a pair of plates Put a pair of plates
 (electrodes) (electrodes)
 in a glass tube in a glass tube
 and apply a several kV and apply a several kV
 high voltage potential high voltage potential
 between them. between them.
 When the gas pressure When the gas pressure
 in the tube in the tube
 becomes very low becomes very low
 (lower than 0.1 (lower than 0.1
 atmospheric pressure), atmospheric pressure),
 an electric discharge an electric discharge
 will take place. will take place.
 This is called This is called
 vacuum discharge. vacuum discharge.
 We can see a striped We can see a striped
 pattern in the tube. pattern in the tube.
 An example is shown in An example is shown in
 the picture below. the picture below.



 When the gas pressure When the gas pressure
 in the tube in the tube
 becomes about becomes about
 0.000001 atmospheric pressure, 0.000001 atmospheric pressure,
 the striped pattern the striped pattern
 will disappear will disappear
 and the inside and the inside
 of the tube will of the tube will
 become dark. become dark.
 However this does However this does
 not mean not mean
 the discharge stopped; the discharge stopped;
 a current is still a current is still
 passing between the electrodes. passing between the electrodes.
 Namely, something is flowing Namely, something is flowing
 between the electrodes. between the electrodes.
 These are called These are called
 the cathode rays. the cathode rays.

 The equipment The equipment
 to generate the cathode rays to generate the cathode rays
 was named was named
 Crookes tube Crookes tube
 after its inventor after its inventor
 W. Crookes W. Crookes
 (UK, 1832 - 1919). (UK, 1832 - 1919).

 In order to investigate In order to investigate
 the properties of the properties of
 cathode rays cathode rays
 we place a cross we place a cross
 in the Crookes tube in the Crookes tube
 as seen in the as seen in the
 following figures following figures
 and set a screen and set a screen
 of a fluorescent substance of a fluorescent substance
 beyond the cross beyond the cross
 also inside the tube. also inside the tube.
 Then we can watch Then we can watch
 a shadow of the cross a shadow of the cross
 on the screen. on the screen.
 This implies This implies
 that the cathode rays that the cathode rays
 emitted from the cathode emitted from the cathode
 toward the anode toward the anode
 travel in straight lines travel in straight lines
 to the screen. to the screen.





|
 [What are the [What are the
 Cathode Rays?] Cathode Rays?]

 J. J. Thomson J. J. Thomson
 (UK, 1856 - 1940) (UK, 1856 - 1940)
 investigated the true nature investigated the true nature
 of cathode rays of cathode rays
 (1897). (1897).

|


|
 There have been There have been
 three famous British physicists three famous British physicists
 named "Thomson" named "Thomson"
 in the history in the history
 of science. of science.
 Do not confuse them. Do not confuse them.

 (1) (1)
 W. Thomson W. Thomson
 (William Thomson, (William Thomson,
 1824 - 1907). 1824 - 1907).
 "Lord Kelvin". "Lord Kelvin".
 The unit of absolute The unit of absolute
 temperature K temperature K
 is given after his name. is given after his name.

 (2) (2)
 J. J. Thomson J. J. Thomson
 (Joseph John Thomson, (Joseph John Thomson,
 1856 - 1940). 1856 - 1940).
 Under consideration Under consideration
 on the present page. on the present page.
 J. J. Thomson had J. J. Thomson had
 many achievements many achievements
 including not only including not only
 the discovery of the electron the discovery of the electron
 but the proposal but the proposal
 of the atomic model of the atomic model
 discussed later discussed later
 and and
 many other discoveries. many other discoveries.

 (3) (3)
 G. P. Thomson G. P. Thomson
 (George Paget Thomson: (George Paget Thomson:
 1892 - 1975) 1892 - 1975)
 A son of the above A son of the above
 J. J. Thomson. J. J. Thomson.
 G. P. Thomson G. P. Thomson
 confirmed the diffraction confirmed the diffraction
 of electrons of electrons
 with the use with the use
 of a metsllic crystal of a metsllic crystal
 to prove the wave to prove the wave
 nature of electrons nature of electrons
 independently of independently of
 the works of US physicists, the works of US physicists,
 C. J. Davisson C. J. Davisson
 and L. H. Germer. and L. H. Germer.

|
 The apparatus used The apparatus used
 by J. J. Thomson by J. J. Thomson
 to investigate to investigate
 the properties of the properties of
 cathode rays cathode rays
 is schematically shown is schematically shown
 in the following in the following
 figure. figure.
 Its basic idea Its basic idea
 is essentailly the same as that is essentailly the same as that
 of the Crookes tube. of the Crookes tube.


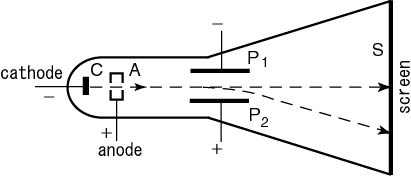
 J. J. Thomson presumed J. J. Thomson presumed
 that the cathode rays that the cathode rays
 that came out of that came out of
 the negative electrode the negative electrode
 (cathode) (cathode)
 were a collection were a collection
 (beam) of (beam) of
 the same particles the same particles
 with negative charges. with negative charges.
 If they are so, If they are so,
 these particles these particles
 emitted from the cathode emitted from the cathode
 would be pulled would be pulled
 by the positive by the positive
 electrode (anode), electrode (anode),
 pass through a small hole pass through a small hole
 at the center at the center
 of the anode, of the anode,
 travel in a straight line, travel in a straight line,
 pass between pass between
 the plates, P1 the plates, P1
 and P2, and P2,
 and finally arrive and finally arrive
 at a screen at a screen
 of fluorescent substance of fluorescent substance
 to make a small spot to make a small spot
 on it. on it.

 If the electric If the electric
 potential is potential is
 so applied so applied
 that the upper plate that the upper plate
 is negative is negative
 and the lower and the lower
 is positive, then is positive, then
 the beam would the beam would
 be curved downward be curved downward
 and the spot on the screen and the spot on the screen
 would move downward. would move downward.
 J. J. Thomson J. J. Thomson
 observed this phenomenon, observed this phenomenon,
 and he also found and he also found
 that the spot neither that the spot neither
 spreaded greatly spreaded greatly
 nor faded. nor faded.

 We can conclude We can conclude
 from these results from these results
 that J. J. Thomson's that J. J. Thomson's
 presumption is correct; presumption is correct;
 namely, the cathode rays namely, the cathode rays
 are a beam are a beam
 of the same kind of of the same kind of
 "particles" "particles"
 with a negative charge. with a negative charge.

|
 [Charge-to-Mass Ratio [Charge-to-Mass Ratio
 of the Cathode Rays] of the Cathode Rays]

 Using the experimental Using the experimental
 set up discussed above, set up discussed above,
 we can measure we can measure
 the charge-to-mass ratio the charge-to-mass ratio
 e /m e /m
 of the "particle" of the "particle"
 in the cathode rays. in the cathode rays.
 Here e is Here e is
 the charge the charge
 of the particle and of the particle and
 m is its mass. m is its mass.
 To explain the To explain the
 details details
 of the method of the method
 to evaluate the to evaluate the
 charge-to-mass ratio, charge-to-mass ratio,
 we need some we need some
 mathematical expressions, mathematical expressions,
 which we which we
 will explain will explain
 on another page: on another page:

 1-7-A:
Measurement of the Charge-to-Mass Ratio of Cathode Rays
1-7-A:
Measurement of the Charge-to-Mass Ratio of Cathode Rays

 Using the method Using the method
 explained in the other page explained in the other page
 (1-7-A), (1-7-A),
 J. J. Thomson measured J. J. Thomson measured
 the charge-to-mass ratio the charge-to-mass ratio
 of the particle of the particle
 in the cathode rays in the cathode rays
 and got a value of and got a value of



 A more precise A more precise
 estimate is estimate is




 J. J. Thomson confirmed J. J. Thomson confirmed
 that an almost that an almost
 constant value constant value
 of e /m of e /m
 was always obtained was always obtained
 under various experimental under various experimental
 conditions. conditions.
 He therefore concluded He therefore concluded
 that cathode that cathode
 rays are a collection rays are a collection
 of the same kind of the same kind
 of particles. of particles.

|
 [The True Nature of [The True Nature of
 the Cathode Rays, the Cathode Rays,
 the Electron] the Electron]

 Let us compare Let us compare
 the charge-to-mass ratio the charge-to-mass ratio
 of the particle of the particle
 in the cathode rays in the cathode rays
 with that of with that of
 the hydrogen ion. the hydrogen ion.

 As seen in As seen in

 Faraday's Law of Electrolysis
Faraday's Law of Electrolysis

 on Page 1-6, on Page 1-6,
 a charge of a charge of

 is necessary to separate is necessary to separate
 1 gram equivalent element 1 gram equivalent element
 through the electrolysis. through the electrolysis.

 Consider the case Consider the case
 of hydrogen for example. of hydrogen for example.
 Because the valence Because the valence
 of hydrogen is 1 of hydrogen is 1
 and the atomic weight and the atomic weight
 of hydrogen is about 1, of hydrogen is about 1,
 1 gram of hydrogen ions 1 gram of hydrogen ions
 have a charge of have a charge of
 . .
 Namely the charge-to-mass ratio Namely the charge-to-mass ratio
 of the hydrogen ion of the hydrogen ion
 is about is about
 . .
 Accordingly, Accordingly,




 This inplies either This inplies either
 that the mass of the that the mass of the
 "particles" in cathode rays "particles" in cathode rays
 is about 1/1800 is about 1/1800
 of the mass of the mass
 of a hydrogen ion, of a hydrogen ion,
 or that the "particle" or that the "particle"
 can carry 1800 times can carry 1800 times
 more charge than more charge than
 a hydrogen ion. a hydrogen ion.
 J. J. Thomson thought J. J. Thomson thought
 that the latter is that the latter is
 not plausible. not plausible.
 Therfore he assumed Therfore he assumed
 the former the former
 and named the particle and named the particle
 in the cathode rays: in the cathode rays:
 electron (1897), electron (1897),
 whose mass is whose mass is
 by a factor of by a factor of
 about 1/1800 than about 1/1800 than
 the lightest atom, the lightest atom,
 hydrogen. hydrogen.

|
 [The Mass of the Electron] [The Mass of the Electron]

 A little later than A little later than
 J. J. Thomson's research J. J. Thomson's research
 on the electron, on the electron,
 the value of the value of
 the elementary charge the elementary charge
 e e
 was clarified was clarified
 by the Millikan oil by the Millikan oil
 drop experiment drop experiment
 as explained on Page 1-6; as explained on Page 1-6;

 Millikan's Experiment
Millikan's Experiment

 Thus it is quite natural Thus it is quite natural
 that the charge of the electron that the charge of the electron
 would be equal would be equal
 to the elementary charge e. to the elementary charge e.
 Accordingly, the mass Accordingly, the mass
 of the electron of the electron
 is determined. is determined.

 Today, the data Today, the data
 on the electron are precisely on the electron are precisely
 measured as measured as







|
 [Electron as a Common [Electron as a Common
 Constituent of Atoms] Constituent of Atoms]


 Various experiments Various experiments
 showed that showed that
 the properties the properties
 of cathode rays of cathode rays
 do not depend do not depend
 on the kind of gas on the kind of gas
 in the discharge tube. in the discharge tube.
 Moreover, when a metal Moreover, when a metal
 is heated to is heated to
 a very high temperature a very high temperature
 around around

 a large number of electrons are a large number of electrons are
 emitted from it. emitted from it.
 They are called They are called
 thermal electrons. thermal electrons.

 O. W. Richardson O. W. Richardson
 (UK, 1879 - 1959) (UK, 1879 - 1959)
 investigated the details investigated the details
 of the thermal electrons. of the thermal electrons.
 He considered He considered
 that the electrons that the electrons
 in atoms in a highly in atoms in a highly
 heated metal heated metal
 were strongly shaken were strongly shaken
 and rushed out of the metal. and rushed out of the metal.
 Thus he confirmed Thus he confirmed
 that the electron that the electron
 is one of the common is one of the common
 constituents of atoms. constituents of atoms.

|
 Top
Top
|
|

 Go back to
the top page of Part 1. Go back to
the top page of Part 1.

 Go back to
the last page. Go back to
the last page.

 Go to
the next page. Go to
the next page.
|