
 Top of Part 1
Top of Part 1

 Last page
Last page

 Next Page
Next Page
  Top of Part 1
Top of Part 1
  Last page
Last page
  Next Page
Next Page
|
1-2: The Discovery of the Atom |


|
 [The Atomism of Demokritos] [The Atomism of Demokritos]

 The idea that The idea that
 the matter consists of the matter consists of
 minimum units, minimum units,
 i.e. atoms, i.e. atoms,
 was proposed by an was proposed by an
 ancient Greek philosopher, ancient Greek philosopher,
 Demokritos Demokritos
 (Ancient Greece, (Ancient Greece,
 ~ B.C. 500). ~ B.C. 500).
 "Atom" means "Atom" means
 "unable to be divided". "unable to be divided".

 Demokritos thought that Demokritos thought that
 this world is made of this world is made of
 the atoms the atoms
 which are moving which are moving
 in the "empty" in the "empty"
 which spread infinitely. which spread infinitely.

 On the other hand, On the other hand,
 Aristoteles Aristoteles
 (Ancient Greece, (Ancient Greece,
 B.C. 384 - 322) B.C. 384 - 322)
 thought that thought that
 the world is filled the world is filled
 with continuous substances. with continuous substances.

 The view of the Aristoteles The view of the Aristoteles
 style was dominant style was dominant
 in a period in a period
 from the ancient from the ancient
 to the medieval times. to the medieval times.

|
 [Existence of [Existence of
 the Chemical Elements] the Chemical Elements]

 In the 18th century, In the 18th century,
 the experimental chemistry the experimental chemistry
 became precise, became precise,
 and then oxygen and hydrogen and then oxygen and hydrogen
 were discovered for example. were discovered for example.
 Thereby Aristoteles' Thereby Aristoteles'
 four-element theory four-element theory
 in which in which
 the world was thought the world was thought
 to be made of the four elements, to be made of the four elements,
 i.e., fire, water, i.e., fire, water,
 earth, and air, earth, and air,
 was denied. was denied.

 A. L. Lavoisier A. L. Lavoisier
 (France, 1743 - 94) (France, 1743 - 94)
 elucidated that elucidated that
 there exist some there exist some
 elements which could elements which could
 not be disintegrated not be disintegrated
 into any fragment into any fragment
 by usual chemical means. by usual chemical means.
 He defined this type of thing as He defined this type of thing as
 "chemical element" "chemical element"
 or simply or simply
 "element" "element"
 (1789). (1789).
 He therefore thought He therefore thought
 that every matter that every matter
 should be made should be made
 of a combination of a combination
 or a compound or a compound
 of various elements. of various elements.

|
 [The Law of [The Law of
 Constant Proportions] Constant Proportions]

 In the 18th In the 18th
 and 19th centuries, and 19th centuries,
 the scientific atomism the scientific atomism
 based on the experimental based on the experimental
 facts was established. facts was established.

 First, the law that First, the law that

|
 "the composition "the composition
 of a pure chemical compound of a pure chemical compound
 is independent of is independent of
 its method of preparation" its method of preparation"

|
 was made clear. was made clear.
 This is called This is called
 the law of constant the law of constant
 proportions. proportions.

 For example, For example,
 although water although water
 is a compound of is a compound of
 two kinds of elements, two kinds of elements,
 hydrogen and oxygen, hydrogen and oxygen,
 the ratio of the ratio of
 the weight of hydrogen the weight of hydrogen
 to that of oxygen to that of oxygen
 in the water in the water
 is fixed at the value is fixed at the value
 1 : 8, 1 : 8,
 independently of independently of
 how it is formed. how it is formed.

|
 [The Law of [The Law of
 Multiple Proportions] Multiple Proportions]

 Furthermore, Furthermore,
 an important law, an important law,
 the law of multiple the law of multiple
 proportions, proportions,
 was proposed. was proposed.

 It states that, It states that,

|
 "When two elements "When two elements
 A and B combine A and B combine
 to form more to form more
 than one compound, than one compound,
 the weights of the weights of
 B which combine B which combine
 with a fixed weight with a fixed weight
 of A are of A are
 in the proportion in the proportion
 of small whole numbers of small whole numbers
 (integers)". (integers)".

|
 For example, For example,
 let's consider let's consider
 the case where carbon the case where carbon
 and oxygen and oxygen
 are combined to form are combined to form
 carbon dioxide carbon dioxide
 and carbon monoxide. and carbon monoxide.
 While 12 g of While 12 g of
 carbon is combined carbon is combined
 with 32 g of oxygen with 32 g of oxygen
 to form carbon dioxide, to form carbon dioxide,
 the same weight the same weight
 of carbon is combined of carbon is combined
 with 16 g of oxygen with 16 g of oxygen
 to form carbon monoxide. to form carbon monoxide.
 Therefore the ratio Therefore the ratio
 of the weights of of the weights of
 oxygen combined with oxygen combined with
 12 g of carbon 12 g of carbon
 is 32 : 16 is 32 : 16
 = 2 : 1. = 2 : 1.

|


|
 [The Atomism of Dalton] [The Atomism of Dalton]

 The above-stated two The above-stated two
 laws may easily laws may easily
 be understood be understood
 if we assume if we assume
 that every element that every element
 consists of consists of
 basic units called atoms. basic units called atoms.

 This is the atomism This is the atomism
 (theory of atom) (theory of atom)
 proposed in 1808 proposed in 1808
 by J. Dalton by J. Dalton
 (UK, 1766 - 1844). (UK, 1766 - 1844).
 It is the first It is the first
 scientific atomism. scientific atomism.

 Let's explain Let's explain
 the theory taking the theory taking
 the case as an example the case as an example
 where carbon combines where carbon combines
 with oxygen with oxygen
 to form carbon dioxide to form carbon dioxide
 and carbon monoxide. and carbon monoxide.
 They combine with each They combine with each
 in the following in the following
 two ways: two ways:



|
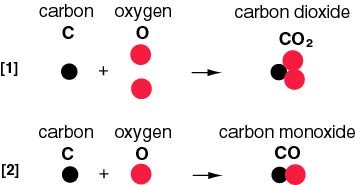
 As shown in As shown in
 the illustration below, the illustration below,
 one carbon atom (C) one carbon atom (C)
 combines with combines with
 two oxygen atoms (O) two oxygen atoms (O)
 in the reaction [1], in the reaction [1],
 on the other hand on the other hand
 one carbon atom (C) one carbon atom (C)
 combines with combines with
 one oxygen atom (O) one oxygen atom (O)
 in the reaction [2]. in the reaction [2].
 Accordingly, Accordingly,
 if we assume if we assume
 that the atomic weight that the atomic weight
 (relative atomic mass) (relative atomic mass)
 of carbon is 12, of carbon is 12,
 then that of oxygen then that of oxygen
 is thought to be 16. is thought to be 16.


 Dalton clarified Dalton clarified
 the combination of atoms the combination of atoms
 in various compounds in various compounds
 as playing as playing
 a jigsaw puzzle a jigsaw puzzle
 or a patchwork. or a patchwork.

 Consequently, Consequently,
 it is considered that it is considered that
 one oxygen atom one oxygen atom
 and two hydrogen atoms and two hydrogen atoms
 are combined are combined
 with each other with each other
 to form water to form water
 as shown in as shown in
 the following the following
 figure. figure.



 Accordingly, Accordingly,
 if we assume if we assume
 the atomic weight the atomic weight
 of oxygen to be 16, of oxygen to be 16,
 then that of hydrogen then that of hydrogen
 is considered to be 1. is considered to be 1.

 Thus the idea Thus the idea
 that the most fundamental that the most fundamental
 units of matter units of matter
 are atoms are atoms
 has been established. has been established.
 This is nothing else This is nothing else
 than the foundation than the foundation
 of the scientific of the scientific
 atomism. atomism.

|
 [Atomic Weight] [Atomic Weight]

 The atomic mass The atomic mass
 measured measured
 in suitable units in suitable units
 is called atomic weight. is called atomic weight.

 As clearly seen As clearly seen
 in Dalton's atomism, in Dalton's atomism,
 if we assume the mass if we assume the mass
 of hydrogen atom to be 1, of hydrogen atom to be 1,
 then that of carbon then that of carbon
 is about 12, is about 12,
 and oxygen to be about 16. and oxygen to be about 16.
 The atomic weight The atomic weight
 of a comparatively of a comparatively
 light element light element
 is close to an integer, is close to an integer,
 but but
 that of a heavier element that of a heavier element
 deviates from an integer. deviates from an integer.

 At present, At present,
 the atomic mass the atomic mass
 is expressed is expressed
 in units of in units of
 atomic mass unit atomic mass unit
 (u). (u).



 An element in nature An element in nature
 is usually is usually
 a mixture of isotopes a mixture of isotopes
 of the element. of the element.
 The number of atoms The number of atoms
 of a given isotope of a given isotope
 in the mixture in the mixture
 is called abundance; is called abundance;
 usually expressed usually expressed
 as a percentage as a percentage
 of the total number of the total number
 of the atoms of the atoms
 of the element. of the element.

 For example, For example,
 the carbon element the carbon element
 contains two kinds of contains two kinds of
 carbon isotopes, carbon isotopes,
 i.e., 12C i.e., 12C
 and 13C; and 13C;
 their abundances their abundances
 are 98.9% are 98.9%
 and 1.1%, and 1.1%,
 respectively. respectively.

 The atomic mass unit The atomic mass unit
 is defined is defined
 as one-twelfth as one-twelfth
 of the mass of of the mass of
 a carbon atom a carbon atom
 of the isotope 12C of the isotope 12C
 which is the most abundant which is the most abundant
 carbon isotope carbon isotope
 in nature. in nature.
 The atomic weights The atomic weights
 are therefore are therefore
 the masses relative the masses relative
 to 12C to 12C
 as 12 [u]. as 12 [u].

 The atomic weight The atomic weight
 of an element of an element
 in nature is in nature is
 the mean value the mean value
 obtained by taking obtained by taking
 the average of the average of
 the atomic masses the atomic masses
 being multiplied being multiplied
 by the corresponding by the corresponding
 abundances. abundances.
 For example, For example,
 the atomic weight the atomic weight
 of natural carbon is of natural carbon is



 Similarly, that of Similarly, that of
 hydrogen is 1.008 [u], hydrogen is 1.008 [u],
 helium 4.003 [u], helium 4.003 [u],
 oxygen 16.00 [u] oxygen 16.00 [u]
 and sodium 22.99 [u]. and sodium 22.99 [u].

|
 Top
Top
|
|

 Go back to
the top page of Part 1. Go back to
the top page of Part 1.

 Go back to
the last page. Go back to
the last page.

 Go to
the next page. Go to
the next page.
|