
 Top of Part 1
Top of Part 1

 Last page
Last page

 Next page
Next page
  Top of Part 1
Top of Part 1
  Last page
Last page
  Next page
Next page
|
1-3: Introduction of the Concept of the Molecule |
 [Molecule] [Molecule]

 The smallest unit The smallest unit
 particle of matter particle of matter
 which has a specified which has a specified
 chemical property is called chemical property is called
 molecule. molecule.

 For example, For example,
 if we divide if we divide
 the water into the water into
 fine portions, fine portions,
 we reach we reach
 the smallest unit the smallest unit
 particles possessing particles possessing
 the property the property
 of water. of water.
 This is the molecule This is the molecule
 of water (H2O). of water (H2O).
 If we divide If we divide
 this molecule of water, this molecule of water,
 we have two hydrogen atoms we have two hydrogen atoms
 and one oxygen atom, and one oxygen atom,
 but they no longer but they no longer
 have the chemical property have the chemical property
 specifying the water. specifying the water.

 The general idea The general idea
 of molecules was of molecules was
 not yet clear not yet clear
 in the atomic theory in the atomic theory
 (atomism) of Dalton. (atomism) of Dalton.
 It was after It was after
 the following the following
 Avogadro's law, Avogadro's law,
 in which the concept in which the concept
 of atom and that of molecule of atom and that of molecule
 were clearly distinguished. were clearly distinguished.

|


|
 [Gay-Lussac's Law, [Gay-Lussac's Law,
 Avogadro's Law] Avogadro's Law]

 J. L. Gay-Lussac J. L. Gay-Lussac
 (France: 1778 - 1850) (France: 1778 - 1850)
 found found
 the following law the following law
 on gaseous reaction (1809): on gaseous reaction (1809):

 For example, For example,
 one volume of one volume of
 nitrogen combines nitrogen combines
 with three volumes with three volumes
 of hydrogen to form of hydrogen to form
 two volumes two volumes
 of ammonia. of ammonia.

 A. Avogadro A. Avogadro
 (Italy, 1776 - 1856) (Italy, 1776 - 1856)
 considered that considered that
 this simple integer ratio this simple integer ratio
 is just the ratio is just the ratio
 of the numbers of molecules of the numbers of molecules
 contained in these gases. contained in these gases.
 Thus he proposed Thus he proposed
 the following the following
 Avogadro's law Avogadro's law
 (1811). (1811).

|

 This law was This law was
 later confirmed later confirmed
 experimentally. experimentally.

 On the basis On the basis
 of this Avogadro's law, of this Avogadro's law,
 it became possible it became possible
 to compare to compare
 the relative weights the relative weights
 of various molecules of various molecules
 and atoms. and atoms.

 The molecule of water The molecule of water
 is expressed as is expressed as
 H2O, H2O,
 and the molecule and the molecule
 of carbon monoxide of carbon monoxide
 is CO is CO
 and that of carbon and that of carbon
 dioxide CO2. dioxide CO2.
 Following the above-mentioned Following the above-mentioned
 Avogadro's law, Avogadro's law,
 we can consider we can consider
 that a gas molecule that a gas molecule
 consisting of consisting of
 a single element, a single element,
 for example like for example like
 a hydrogen molecule a hydrogen molecule
 or an oxygen molecule, or an oxygen molecule,
 is a composite is a composite
 of two atoms of two atoms
 which could be expressed which could be expressed
 as H2 as H2
 or O2. or O2.
 This type of molecule This type of molecule
 is called is called
 diatomic molecule. diatomic molecule.

 Therefore the reaction Therefore the reaction
 in which hydrogen in which hydrogen
 and oxygen and oxygen
 combine to form water combine to form water
 can be displayed as can be displayed as
 the following the following
 figure. figure.


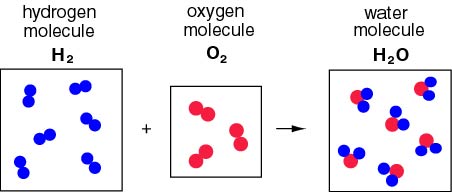
 Since the molecule Since the molecule
 of the noble gases of the noble gases
 (helium, neon, (helium, neon,
 argon, krypton, argon, krypton,
 xenon and radon) xenon and radon)
 consists of single atoms, consists of single atoms,
 it is called it is called
 monoatomic molecule. monoatomic molecule.
 "Inert gases" "Inert gases"
 is a semi-obsolete name is a semi-obsolete name
 for the noble gases. for the noble gases.

|
 [Molecular Weight] [Molecular Weight]

 The mass of a molecule, The mass of a molecule,
 namely the namely the
 molecular weight, molecular weight,
 is equal is equal
 to the sum to the sum
 of the atomic weights of the atomic weights
 of the constituent atoms. of the constituent atoms.
 For example, For example,
 the molecule the molecule
 of water is H2O, of water is H2O,
 and the atomic weight and the atomic weight
 of hydrogen H is of hydrogen H is
 about 1 [u] about 1 [u]
 and that of oxygen O and that of oxygen O
 is about 16 [u]. is about 16 [u].
 Accordingly, Accordingly,
 the molecular weight the molecular weight
 of water is of water is

 1 x 2 + 16 = 18 [u]. 1 x 2 + 16 = 18 [u].

|
 [Avogadro's Constant] [Avogadro's Constant]


 The amount of The amount of
 a compound a compound
 or an element or an element
 which is numerically which is numerically
 equal to the molecular equal to the molecular
 weight in grams weight in grams
 is called is called
 mole expressed mole expressed
 by the symbol "mol". by the symbol "mol".
 The mole is also The mole is also
 called gram molecule. called gram molecule.

 Since the molecular Since the molecular
 weight of carbon 12 weight of carbon 12
 (12C) (12C)
 is, for example, is, for example,
 12 [u], 12 [u],
 1 mole 1 mole
 (= 1 gram molecule) (= 1 gram molecule)
 of carbon 12 of carbon 12
 is 12 grams of it. is 12 grams of it.

 At "stp" At "stp"
 that denotes that denotes
 the "standard temperature the "standard temperature
 and pressure", and pressure",
 the volume of gas the volume of gas
 of 1 mol of 1 mol
 is 22.414 liter is 22.414 liter
 being independent being independent
 of the kind of gas. of the kind of gas.

 The number of molecules The number of molecules
 in one mole of in one mole of
 any pure substance any pure substance
 is a constant is a constant
 called called
 Avogadro's constant Avogadro's constant
 NA, NA,
 which has formerly which has formerly
 been called been called
 "Avogadro's number". "Avogadro's number".
 It has been determined It has been determined
 by various methods by various methods
 and the result is and the result is



|
 Top
Top
|
|

 Go back to
the top page of Part 1. Go back to
the top page of Part 1.

 Go back to
the last page. Go back to
the last page.

 Go to
the next page. Go to
the next page.
|