
 Top of Part 2
Top of Part 2

 Last page
Last page

 Next page
Next page
  Top of Part 2
Top of Part 2
  Last page
Last page
  Next page
Next page
|
2-6: What is the Atomic Nucleus? |
 As discussed As discussed
 in the preceding page, in the preceding page,
 2-5, 2-5,
 the success of the success of
 the Rutherford model the Rutherford model
 of the nuclear atom of the nuclear atom
 taught us that taught us that
 an atom consists an atom consists
 of the positively of the positively
 charged massive nucleus charged massive nucleus
 and the surrounding and the surrounding
 light electrons. light electrons.

 An atom contains An atom contains
 Z electrons Z electrons
 whose total charge is whose total charge is
 -Ze, -Ze,
 so that the positive charge so that the positive charge
 +Ze cancelling +Ze cancelling
 this negative charge this negative charge
 of the electrons of the electrons
 is considered is considered
 to be concentrated to be concentrated
 into the nucleus. into the nucleus.

 It has been explained It has been explained
 in detail in detail
 in the previous pages in the previous pages
 that that
 the total mass the total mass
 of the electrons of the electrons
 in an atom is in an atom is
 extremely smaller extremely smaller
 than the total mass than the total mass
 of the atom. of the atom.
 This means that This means that
 almost all mass of an atom almost all mass of an atom
 is carried by the nucleus. is carried by the nucleus.

 Then, Then,
 what is an atomic what is an atomic
 nucleus made of? nucleus made of?
 What are the constituents What are the constituents
 of the nucleus? of the nucleus?
 What is the structure What is the structure
 of the nucleus? of the nucleus?

|
 [The Size of a Nucleus] [The Size of a Nucleus]

 Rutherford's formula Rutherford's formula
 of angular distribution of angular distribution
 shown in the preceding page shown in the preceding page
 is obtained is obtained
 by assuming that by assuming that
 the charge of the nucleus the charge of the nucleus
 is concentrated is concentrated
 on a point, on a point,
 i.e. a point charge. i.e. a point charge.
 However, even if the charge However, even if the charge
 spreads over an area spreads over an area
 narrower than the minimum of narrower than the minimum of
 the closest approach the closest approach
 shown in the following shown in the following
 figure, figure,
 Rutherford's formula Rutherford's formula
 must still hold. must still hold.
 Therefore, if the experimental Therefore, if the experimental
 data are well reproduced data are well reproduced
 by Rutherford's formula, by Rutherford's formula,
 the nuclear radius the nuclear radius
 of that atom of that atom
 is understood to be is understood to be
 smaller than smaller than
 the minimum of the minimum of
 the closest approach. the closest approach.

 Let us look back Let us look back
 the trajectories the trajectories
 of Rutherford scattering of Rutherford scattering
 shown in shown in
 the following figure, the following figure,
 where the red arrow where the red arrow
 at the central part at the central part
 denotes the minimum of denotes the minimum of
 the closest approach. the closest approach.

|
|



 In the experiment In the experiment
 of the alpha particle of the alpha particle
 scattering scattering
 for the target for the target
 of a copper foil, of a copper foil,
 the resultant data the resultant data
 are well fit are well fit
 to Rutherford's to Rutherford's
 formula up to formula up to
 the scattering angle the scattering angle

 The incident energy The incident energy
 in this case is in this case is
 E = 5.3 MeV, E = 5.3 MeV,
 and the atomic number and the atomic number
 of copper is of copper is
 Z = 29. Z = 29.
 The minimum of The minimum of
 the closest approach the closest approach
 is given by is given by



 Consequently, Consequently,
 we can say we can say
 that the radius that the radius
 of the copper nucleus of the copper nucleus
 is less than is less than

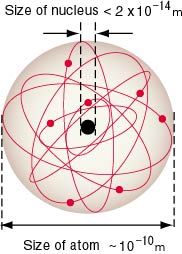
 Comparing this nuclear Comparing this nuclear
 radius with that of radius with that of
 an atom, an atom,

 the size of a nucleus the size of a nucleus
 is less than 1/5000 is less than 1/5000
 of that of an atom. of that of an atom.
 You must be able You must be able
 to realize to realize
 how small how small
 the atomic nucleus is. the atomic nucleus is.

|
 [Artificial Transmutation [Artificial Transmutation
 of Elements, Proton] of Elements, Proton]

 In 1919, In 1919,
 Rutherford succeeded Rutherford succeeded
 in changing nitrogen in changing nitrogen
 into oxygen into oxygen
 artificially. artificially.
 This was the first This was the first
 artificial transmutation artificial transmutation
 of elements. of elements.

 As part of As part of
 the alpha particle the alpha particle
 scattering experiment, scattering experiment,
 Rutherford placed Rutherford placed
 a radioactive material a radioactive material
 in nitrogen gas in nitrogen gas
 as shown in the following as shown in the following
 figure, figure,
 and he observed the and he observed the
 scintillations on the scintillations on the
 fluorescent substance. fluorescent substance.
 He found very He found very
 bright scintillations bright scintillations
 whose directions whose directions
 were not considered were not considered
 to be those by alpha particles. to be those by alpha particles.




 Similar phenomena Similar phenomena
 in various kinds in various kinds
 of elements, of elements,
 for example, for example,
 boron, fluorine, boron, fluorine,
 neon, sodium, neon, sodium,
 phosphorus, sulfur, phosphorus, sulfur,
 argon, and so on argon, and so on
 were observed later. were observed later.

 These phenomena These phenomena
 were considered that were considered that
 high speed alpha high speed alpha
 particles collide particles collide
 with atoms and knock with atoms and knock
 on an "unknown" particle on an "unknown" particle
 to rush out to rush out
 with high energies. with high energies.

 Photographs of Photographs of
 these phenomena these phenomena
 were successfully were successfully
 taken with taken with
 Wilson's cloud chamber.
Wilson's cloud chamber.

 By putting the cloud chamber By putting the cloud chamber
 in a magnetic field in a magnetic field
 and analyzing and analyzing
 the results precisely, the results precisely,
 it was clarified it was clarified
 that these "unknown" that these "unknown"
 particles are particles are
 the same as the same as
 the hydrogen ions. the hydrogen ions.

 Since a hydrogen atom Since a hydrogen atom
 consists of an electron consists of an electron
 and a nucleus, and a nucleus,
 a hydrogen ion a hydrogen ion
 is the hydrogen atom is the hydrogen atom
 having lost an electron. having lost an electron.
 This is nothing else This is nothing else
 than the than the
 nucleus of hydrogen atom, nucleus of hydrogen atom,
 which was named which was named
 proton proton
 by Rutherford. by Rutherford.

 This phenomenon This phenomenon
 was considered was considered
 as the following process: as the following process:




 This means This means
 that the nucleus that the nucleus
 of the nitrogen atom of the nitrogen atom
 is disintegrated is disintegrated
 under the strong forces under the strong forces
 exerted exerted
 by a high speed by a high speed
 alpha particle, alpha particle,
 the hydrogen nucleus the hydrogen nucleus
 (proton) is (proton) is
 liberated, liberated,
 and the rests and the rests
 are rearranged are rearranged
 to form the nucleus to form the nucleus
 of oxygen. of oxygen.
 Namely, it implies Namely, it implies
 that Rutherford that Rutherford
 carried out carried out
 the first artificial the first artificial
 transmutation of element; transmutation of element;
 this is a realization this is a realization
 of alchemists' dream of alchemists' dream
 in a modern form. in a modern form.

|
 [The Constituents of Atomic Nuclei] [The Constituents of Atomic Nuclei]

 Thus, Thus,
 it turned out it turned out
 that the proton that the proton
 is one of the constituents is one of the constituents
 of atomic nuclei. of atomic nuclei.
 However, it was not clear However, it was not clear
 whether a nucleus whether a nucleus
 is made only of protons. is made only of protons.
 It appears quite It appears quite
 reasonable to consider reasonable to consider
 that the helium nucleus that the helium nucleus
 (alpha particle) (alpha particle)
 consists of consists of
 four protons four protons
 and two electrons. and two electrons.
 On the early stage, On the early stage,
 people thought people thought
 that the nucleus that the nucleus
 is made of protons is made of protons
 and electrons. and electrons.
 It has however been It has however been
 clarified that this idea has clarified that this idea has
 a serious inconsistency. a serious inconsistency.

 After the discovery After the discovery
 of the neutron of the neutron
 by J. Chadwick by J. Chadwick
 (UK, 1891 - 1974), (UK, 1891 - 1974),
 it has finally it has finally
 elucidated that elucidated that
 the true constituents the true constituents
 of nuclei of nuclei
 are protons are protons
 and neutrons. and neutrons.

|
 Top
Top
|
|

 Go back to
the top page of Part 2. Go back to
the top page of Part 2.

 Go back to
the last page. Go back to
the last page.

 Go to
the next page. Go to
the next page.
|