
 Epilogue : Opening to Quantum Mechanics
Epilogue : Opening to Quantum Mechanics


|
Microscopic World -1-
(Mysteries of Atomic World)
 Epilogue : Opening to Quantum Mechanics
Epilogue : Opening to Quantum Mechanics
|
 Go back to
the top page of Microscopic World -1- Go back to
the top page of Microscopic World -1-
|
 Throughout all the pages of Throughout all the pages of
 the present Seminar, the present Seminar,
 Microscopic World -1-, Microscopic World -1-,
 we have learned that we have learned that
 matter and matter and
 light in the light in the
 microscopic world microscopic world
 possess both the possess both the
 particle nature and the particle nature and the
 wave nature. wave nature.

 In addition, In addition,
 we could understand we could understand
 that the Bohr model of that the Bohr model of
 atomic structure atomic structure
 is constructed is constructed
 by considering by considering
 both the natures. both the natures.
 By using this model, By using this model,
 it has become possible it has become possible
 to explain the stability to explain the stability
 of atoms and of atoms and
 the atomic spectra the atomic spectra
 which was impossible which was impossible
 to be explained with the to be explained with the
 classical theory classical theory
 (Newtonian mechanics (Newtonian mechanics
 and Maxwellian electromagnetism). and Maxwellian electromagnetism).

 It seems that It seems that
 the double nature the double nature
 (the duality) (the duality)
 of matter and light, of matter and light,
 i.e. the wave i.e. the wave
 and particle natures, and particle natures,
 is a very fundamental is a very fundamental
 property of "things" property of "things"
 or "beings" or "beings"
 existing in our world. existing in our world.
 In other word, In other word,
 the duality seems the duality seems
 to be a fundamental philosophy to be a fundamental philosophy
 for the "existence" for the "existence"
 of things. of things.
 This looks quite This looks quite
 strange and mysterious strange and mysterious
 for us, for us,
 because we are very much because we are very much
 used to the thinking way used to the thinking way
 in the classical theory. in the classical theory.

|
 [Young's Experiment] [Young's Experiment]

 Let us look back Let us look back
 at Young's experiment at Young's experiment
 by which the wave nature by which the wave nature
 of light was confirmed of light was confirmed
 for the first time. for the first time.
 For the sake For the sake
 of convenience, of convenience,
 the contents of the contents of
 Young's experiment Young's experiment
 presented in the page presented in the page
 3-8: Summary of Part 3
3-8: Summary of Part 3
 are shown here again. are shown here again.

|

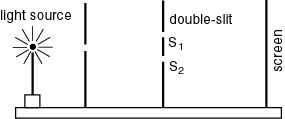
|
Schematic drawing of Young's experiment

 The monochromatic light The monochromatic light
 from the source passes from the source passes
 through the two slits, through the two slits,
 S1 S1
 and and
 S2, S2,
 and produce an interference and produce an interference
 pattern (fringes) pattern (fringes)
 on the screen. on the screen.

|
 An example of An example of
 the picture of the pattern the picture of the pattern
 is represented in is represented in
 the following picture (A) the following picture (A)
 and (B). and (B).
 In these pictures, In these pictures,
 (A) shows the result when (A) shows the result when
 one of the slits is closed, one of the slits is closed,
 and (B) the result when and (B) the result when
 both of the slits are opened. both of the slits are opened.




 We will discuss We will discuss
 on some details on some details
 of these pictures below. of these pictures below.
 Before that, Before that,
 let us study let us study
 a little about a little about
 the relation between the basic the relation between the basic
 mechanism of mechanism of
 photography photography
 and the particle nature and the particle nature
 of light. of light.

 [The Mechanism of Photography [The Mechanism of Photography
 and the Light Particle] and the Light Particle]

 In Young's experiment, In Young's experiment,
 the reason that the reason that
 the photographic plate the photographic plate
 (or film) (or film)
 can response can response
 to light is due to to light is due to
 the particle nature of light. the particle nature of light.
 Let us explain this below. Let us explain this below.

 Photography is based Photography is based
 on the chemical reactions on the chemical reactions
 brought about brought about
 by the action of light by the action of light
 (photochemical reactions). (photochemical reactions).
 Namely, the molecules Namely, the molecules
 of halogenated silver of halogenated silver
 are dissociated are dissociated
 by the illumination by the illumination
 of light of light
 and silver atoms and silver atoms
 are educed. are educed.
 For example, For example,
 silver bromide silver bromide
 (AgBr) that is (AgBr) that is
 one of the halogenated silvers one of the halogenated silvers
 is dissociated by light as is dissociated by light as


 AgBr AgBr
 --> -->
 Ag + Br Ag + Br

 to be silver to be silver
 and bromine atoms. and bromine atoms.
 After being treated After being treated
 with special chemicals, with special chemicals,
 only the silver atoms only the silver atoms
 remain to be black coloured remain to be black coloured
 and to make and to make
 a photographic negative. a photographic negative.

 To dissociate a molecule To dissociate a molecule
 of halogenated silver, of halogenated silver,
 roughly speaking, roughly speaking,
 more energy than more energy than
 about 2 eV about 2 eV
 is necessary. is necessary.
 Namely, one molecule Namely, one molecule
 of silver bromide of silver bromide
 (AgBr) (AgBr)
 or silver iodide or silver iodide
 (AgI) (AgI)
 can be dissociated can be dissociated
 only by the accumulation only by the accumulation
 of more energy than of more energy than
 about 2 eV about 2 eV
 This amount of energy This amount of energy
 is almost the same as is almost the same as
 the value of the the value of the
 work function work function
 which is the which is the
 necessary minimum energy necessary minimum energy
 to bring about to bring about
 the photoelectric effect. the photoelectric effect.
 (Refer to the page (Refer to the page
 3-6: The Hypothesis of Light Quanta and the Photoelectric Effect.)
3-6: The Hypothesis of Light Quanta and the Photoelectric Effect.)

 Hence, the discussion Hence, the discussion
 on that page on that page
 about the time about the time
 to make the photoelectric effect to make the photoelectric effect
 take place take place
 is valid for the present is valid for the present
 photochemical reaction photochemical reaction
 as well; as well;
 i.e., if we consider i.e., if we consider
 the time necessary the time necessary
 for the dissociation for the dissociation
 of the halogenated silver of the halogenated silver
 within the classical theory, within the classical theory,
 it should take it should take
 a time longer a time longer
 than the shutter speed than the shutter speed
 of an ordinary camera of an ordinary camera
 that is usually shorter that is usually shorter
 than 1/100 s or 1/1000 s. than 1/100 s or 1/1000 s.
 Consequently, Consequently,
 we cannot help we cannot help
 considering that considering that
 light can dissociate light can dissociate
 the halogenated the halogenated
 silver molecules silver molecules
 being absorbed instantly being absorbed instantly
 as a particle of light as a particle of light
 (photon). (photon).

|
 [The Mysterious Particle-Wave Duality] [The Mysterious Particle-Wave Duality]

 The image of The image of
 the interference fringes the interference fringes
 recorded on the recorded on the
 photographic plate photographic plate
 (film) (film)
 is consists of is consists of
 huge number of silver atoms huge number of silver atoms
 educed by the photochemical educed by the photochemical
 dissociation of dissociation of
 halogenated silver molecules. halogenated silver molecules.
 These silver atoms collect These silver atoms collect
 together to make the together to make the
 interference pattern interference pattern
 on the film. on the film.
 We recognize this collection We recognize this collection
 of the silver atoms of the silver atoms
 as a picture of the as a picture of the
 interference pattern. interference pattern.
 Namely, we are looking at Namely, we are looking at
 the collection of the collection of
 marks or marks or
 traces of photons. traces of photons.
 Each mark denotes Each mark denotes
 a point where a point where
 a particle of light a particle of light
 (photon) has struck. (photon) has struck.
 The collection The collection
 of these huge number of these huge number
 of marks of marks
 constitutes a interference constitutes a interference
 fringes which characterize fringes which characterize
 the wave nature of light. the wave nature of light.
 How can we understand How can we understand
 this mechanism? this mechanism?

 If light were If light were
 simply particles, simply particles,
 one light particle one light particle
 could not pass through could not pass through
 two slits two slits
 simultaneously, simultaneously,
 so that it could never so that it could never
 produce interference fringes. produce interference fringes.
 If light were simple If light were simple
 particles, particles,
 then the picture then the picture
 on the screen on the screen
 should be what should be what
 is obtained by superposing is obtained by superposing
 such a picture such a picture
 in the case of in the case of
 a single slit opened a single slit opened
 as shown in as shown in
 the above picture (A) the above picture (A)
 with another picture with another picture
 obtained by displacing it obtained by displacing it
 by the interslit by the interslit
 distance d. distance d.
 However, we have However, we have
 an interference pattern an interference pattern
 as shown in as shown in
 the picture (B) the picture (B)
 in practice. in practice.
 Hence, we must consider Hence, we must consider
 that light is not that light is not
 a simple particle, a simple particle,
 but possesses some kind but possesses some kind
 of wave nature as well. of wave nature as well.

 If light were If light were
 pure waves, pure waves,
 we could not have we could not have
 a picture of a picture of
 the interference fringes. the interference fringes.
 Because light has Because light has
 the duality the duality
 of wave nature of wave nature
 and particle nature and particle nature
 simultaneously, simultaneously,
 we can have the picture we can have the picture
 of the interference pattern. of the interference pattern.
 We therefore cannot We therefore cannot
 deny the dual deny the dual
 nature of light. nature of light.
 Then, what part of light Then, what part of light
 is a wave? is a wave?
 And what part And what part
 of light is a particle? of light is a particle?

 You might suspect that You might suspect that
 two different photons two different photons
 interfere with each other interfere with each other
 after they pass through after they pass through
 two slits separately two slits separately
 and consequently and consequently
 they produce the they produce the
 interference pattern interference pattern
 on the screen. on the screen.
 However this idea However this idea
 is not valid is not valid
 by the following reason: by the following reason:
 If the intensity If the intensity
 of the incident light of the incident light
 to the double-slit to the double-slit
 experiment is so weak experiment is so weak
 that only one photon that only one photon
 runs at every runs at every
 moment of time, moment of time,
 two different photons two different photons
 are impossible are impossible
 to pass through two slits to pass through two slits
 at one time at one time
 and and
 no interference occurs no interference occurs
 among different photons. among different photons.
 In spite of such extreme In spite of such extreme
 weakness of the intensity weakness of the intensity
 of the incident light, of the incident light,
 the same interference pattern the same interference pattern
 is still obtained is still obtained
 on the photographic plate on the photographic plate
 by exposing it for by exposing it for
 an extremely long time. an extremely long time.
 Such an experiment of Such an experiment of
 three-month exposure time three-month exposure time
 was carried out was carried out
 in 1909 by a British student, in 1909 by a British student,
 and he got a clear and he got a clear
 interference pattern interference pattern
 that is exactly the same as that is exactly the same as
 those in usual those in usual
 Young's experiment. Young's experiment.
 This tells us that This tells us that
 the interference pattern the interference pattern
 in Young's experiment in Young's experiment
 is not caused is not caused
 by the interference by the interference
 between different photons. between different photons.
 Then, why does the Then, why does the
 interference pattern interference pattern
 come into existence? come into existence?

|
 [Opening to Quantum Mechanics] [Opening to Quantum Mechanics]

 As mentioned above, As mentioned above,
 we have encountered we have encountered
 a serious discrepancy a serious discrepancy
 between between
 the particle nature the particle nature
 and the wave nature and the wave nature
 of light. of light.
 This discrepancy This discrepancy
 is not only is not only
 for light but also for light but also
 for electrons. for electrons.

 In order to perfectly In order to perfectly
 overcome this discrepancy, overcome this discrepancy,
 people have had people have had
 to construct a completely to construct a completely
 new theory new theory
 getting over the getting over the
 difficulties in the difficulties in the
 classical theory. classical theory.
 This is nothing else This is nothing else
 than Quantum Mechanics than Quantum Mechanics
 proposed by proposed by
 W. K. Heisenberg W. K. Heisenberg
 (Germany, 1901 - 76) (Germany, 1901 - 76)
 and and
 E. Schroedinger E. Schroedinger
 (Austria, 1887 - 1961). (Austria, 1887 - 1961).

 By this quantum mechanics, By this quantum mechanics,
 the mystery of the particle the mystery of the particle
 and wave natures and wave natures
 of light and electrons of light and electrons
 were solved and were solved and
 the atomic structure the atomic structure
 was clarified. was clarified.
 You are strongly expected You are strongly expected
 to challenge to study to challenge to study
 Quantum Mechanics Quantum Mechanics
 proceeding to proceeding to
 the Second Part of the Second Part of
 the present seminar, the present seminar,

 Microscopic World -2- (Introduction to Quantum Mechanics).
Microscopic World -2- (Introduction to Quantum Mechanics).

|
 Top
Top
|
|

 Go back to Go back to
 the top page of Microscopic World -1-
the top page of Microscopic World -1-
|