
 Top of Part 3
Top of Part 3

 Last page
Last page

 Next page
Next page
  Top of Part 3
Top of Part 3
  Last page
Last page
  Next page
Next page
|
3-2: The Nuclear Force |
 On the preceding page, On the preceding page,
 we learned that we learned that
 the origin of the huge amount the origin of the huge amount
 of nuclear energy of nuclear energy
 is the binding energy is the binding energy
 of nuclei. of nuclei.

 Then, what is the Then, what is the
 binding energy of nuclei? binding energy of nuclei?
 It has been explained It has been explained
 in detail in detail
 on the page, on the page,
 2-3:
"Mass of Nuclei, Binding Energy".
2-3:
"Mass of Nuclei, Binding Energy".
 We can review it We can review it
 as follows: as follows:

 The atomic nucleus consists The atomic nucleus consists
 of many nucleons of many nucleons
 (protons and neutrons) (protons and neutrons)
 which bind to each other which bind to each other
 through the interaction through the interaction
 called nuclear force. called nuclear force.
 The mass of a nucleus The mass of a nucleus
 is slightly less is slightly less
 than the sum of masses than the sum of masses
 of its constituent nucleons. of its constituent nucleons.
 This mass difference This mass difference
 called the mass defect. called the mass defect.
 According to According to
 Einstein's Mass-Energy Equivalence,
Einstein's Mass-Energy Equivalence,
 the mass defect the mass defect
 is converted into an energy. is converted into an energy.
 This is This is
 the binding energy the binding energy
 of the nucleus. of the nucleus.
 The strength of its binding The strength of its binding
 is therefore represented by is therefore represented by
 the magnitude of the magnitude of
 the binding energy. the binding energy.

 Hence, we can say Hence, we can say
 that the final origin of that the final origin of
 the origin of the nuclear energy the origin of the nuclear energy
 is nothing but is nothing but
 the nuclear force. the nuclear force.

 Then, what is the nuclear force? Then, what is the nuclear force?

|
 [The Origin of the Coulomb force] [The Origin of the Coulomb force]

 The Coulomb force The Coulomb force
 works between two electric charges, works between two electric charges,
 q q
 and and
 q'. q'.
 If the two charges are of If the two charges are of
 the same sign, the same sign,
 it is repulsive. it is repulsive.
 If they are of opposite sign, If they are of opposite sign,
 then they attract each other. then they attract each other.

 In the Quantum Mechanics In the Quantum Mechanics
 of Electromagnetic Fields of Electromagnetic Fields
 (or Quantum Electrodynamics), (or Quantum Electrodynamics),
 the exchange of photons the exchange of photons
 between two charges between two charges
 causes causes
 the Coulomb force the Coulomb force
 between them. between them.
 (See the following figure.) (See the following figure.)

|

|
 [The Origin of the Coulomb Force] [The Origin of the Coulomb Force]

 If two charges, If two charges,
 q q
 and and
 q' q'
 , exchange photons, , exchange photons,
 the Coulomb force the Coulomb force
 occurs between them. occurs between them.
 This figure is its schematic This figure is its schematic
 drawing. drawing.
 The solid lines represent The solid lines represent
 particles running upward. particles running upward.

|


|
 [Yukawa's Meson Theory] [Yukawa's Meson Theory]

 In 1935, H. Yukawa In 1935, H. Yukawa
 (Japan, 1907 - 81) (Japan, 1907 - 81)
 proposed a theory for the proposed a theory for the
 origin of the nuclear force origin of the nuclear force
 on an analogy with the above-mentioned on an analogy with the above-mentioned
 interpretation of the Coulomb force. interpretation of the Coulomb force.
 He thought that He thought that
 the nuclear force the nuclear force
 might be caused by might be caused by
 the exchange of some massive the exchange of some massive
 unknown particles unknown particles
 between nucleons. between nucleons.
 Now this particle Now this particle
 is known as is known as
 the pi-meson the pi-meson
 or pion. or pion.
 (See the following figure.) (See the following figure.)

 The pion was found The pion was found
 in cosmic rays in cosmic rays
 by C. F. Powell by C. F. Powell
 (UK, 1903 - 69) et al. (UK, 1903 - 69) et al.
 in 1947 in 1947
 and produced artificially and produced artificially
 by an accelerator in 1948. by an accelerator in 1948.

|
 If pions are exchanged If pions are exchanged
 between two nucleons, between two nucleons,
 the nuclear force the nuclear force
 occurs. occurs.
 (See the following figure.) (See the following figure.)


 There exist There exist
 three types of pions, three types of pions,

 with charge 0, with charge 0,

 with charge +e, and with charge +e, and

 with charge -e. with charge -e.
 Their masses are Their masses are
 nowadays known well as nowadays known well as


|
 [Properties of the Nuclear Force] [Properties of the Nuclear Force]

 The nuclear force is The nuclear force is
 one of the strong interactions. one of the strong interactions.
 It is about ten times It is about ten times
 as strong as the Coulomb force. as strong as the Coulomb force.
 However it is of very short-range However it is of very short-range
 compared with the Coulomb force; compared with the Coulomb force;
 namley, the nuclear force is namley, the nuclear force is
 very strong but it does not work very strong but it does not work
 unless two nucleons approach unless two nucleons approach
 to each other to each other
 in a very short distance. in a very short distance.
 Moreover, it is known that, Moreover, it is known that,
 when they approach when they approach
 very close to each other, ( very close to each other, (
 ), ),

 an extremely strong repulsive force an extremely strong repulsive force
 works. works.

 The detailed properties The detailed properties
 of the nuclear force of the nuclear force
 at the distance smaller than at the distance smaller than

 are not sufficiently clarified yet. are not sufficiently clarified yet.

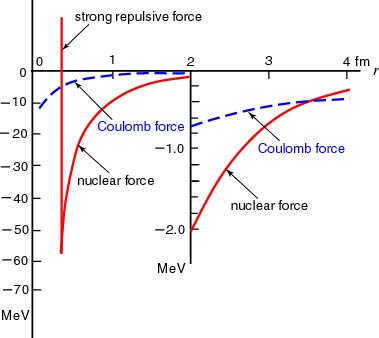
 In the following figure, In the following figure,
 the potential of the nuclear the potential of the nuclear
 force between two nucleons force between two nucleons
 is roughly compared is roughly compared
 with the Coulomb potential with the Coulomb potential
 between two charges, between two charges,
 e e
 and and
 -e. -e.

|
|
 [Comparison between the Nuclear Force [Comparison between the Nuclear Force
 and the Coulomb Force] and the Coulomb Force]
 A rough comparison A rough comparison
 between the nuclear force between the nuclear force
 (between two nucleons) (between two nucleons)
 and the Coulomb force and the Coulomb force
 (between charges (between charges
 e e
 and and
 -e -e
 ) is shown. ) is shown.
 The red solid The red solid
 curve indicates the curve indicates the
 nuclear force nuclear force
 and and
 the blue dashed the blue dashed
 curve the curve the
 Coulomb force. Coulomb force.
 The abscisa denotes the distance The abscisa denotes the distance
 between particles, r. between particles, r.
 In the region of In the region of
 r < 3 fm, r < 3 fm,
 the nuclear force is the nuclear force is
 overwhelmingly stronger than overwhelmingly stronger than
 the Coulomb force. the Coulomb force.
 You should notice that, You should notice that,
 in the very inner region ( in the very inner region (
 r r
 <
<  ), ),
 an extremely strong an extremely strong
 repulsive force works. repulsive force works.

|
 Top
Top
|
|

 Go back to
the top page of Part 3. Go back to
the top page of Part 3.

 Go back to
the last page. Go back to
the last page.

 Go to
the next page. Go to
the next page.
|